M. Esbri-Victor1, I. Huedo-Rodenas1, M. Lopez-Utiel1, J.L. Navarro-Lopez1, M. Martinez-Reig1, J.A. Serra-Rexach2,3, L. Romero-Rizos1,2, P. Abizanda1,2
1. Geriatrics Department. Complejo Hospitalario Universitario de Albacete, Albacete, Spain; 2. CIBERFES, Instituto de Salud Carlos III, Madrid, Spain; 3. Geriatric Department. Hospital General Universitario Gregorio Marañón, Madrid, Spain.
Corresponding author: Pedro Abizanda, MD, PhD. Geriatrics Department. Complejo Hospitalario Universitario de Albacete, 02006 Albacete, Spain. e-mail: pabizanda@sescam.jccm.es, Tfn: +34967597651; Fax : +34967597635.
Care Weekly 2017;1:50-55
Published online November 9, 2017, http://dx.doi.org/10.14283/cw.2017.9
Please, note that this editorial was published also in the Journal of Frailty Aging (JFA) http://dx.doi.org/10.14283/jfa.2017.19
Abstract
Objective: To analyze the association between frailty and Fear of Falling (FoF) in a cohort of older adults with previous falls. Design: Cross-sectional study (FISTAC). Setting: Falls Unit, Complejo Hospitalario Universitario of Albacete (Spain). Participants: 183 adults older than 69 years, from the Falls Unit, with a history of a previous fall in the last year. Measurements: FoF was assessed at baseline using the Falls Efficacy Scale International (FES-I) and three questions previously validated. Frailty was assessed with the frailty phenotype criteria. Age, gender, comorbidity, nutritional status, cognitive status and risk of depression were determined. Results: Mean age 78.4, 80.3% women. FoF was present in 140 (76.5%) participants with the three questions and 102 (55.7%) presented high concern of falling with the FES-I. 88.8% of frail older adults presented FoF compared to 62.4% of those who were not frail, and only 37.8% of non frail had a high concern of falling, compared to 77.2% of those who were frail measured with the FES-I. Frail participants had an adjusted risk of FoF that was 3.18 (95% CI 1.32 to 7.65) higher compared to those who were not frail assessed with the three questions and 3.93 (95% CI 1.85 to 8.36) higher concern of falling when using the FES-I scale. Only female sex and depression risk were also associated to FoF in the final adjusted models. Conclusion: Frailty is independently associated with the FoF syndrome in older faller subjects.
Key words: Frail elderly, fear of falling, falls, older adults.
Introduction
Physical frailty is a medical syndrome with multiple causes and contributors that is characterized by diminished strength, endurance, and reduced physiologic function that increases an individual’s vulnerability for developing increased dependency and/or death (1). Frailty is a common syndrome in community-dwelling older adults with a pooled prevalence of 10.7% (2), and a it has been associated with health related adverse events like mortality, disability in basic activities of daily living (BADL) and mobility disability, hospitalization, institutionalization and falls (3).
The Fear of Falling (FoF) syndrome refers to the lack of self-confidence that normal activities can be performed without falling (4). It has been identified as a common problem affecting between 20.8 and 80% of community-dwelling older adults (5-8), and may be associated with a history of previous falls (6,9), although it can also be present in older adults without previous falls (8,10). Individual factors associated with FoF are older age (6,9), female sex (5,8), polypharmacy (6), obesity (7), vision problems (11), balance and mobility impairment (7,11), activity levels (12), social isolation and living alone (7), low self-rated health (11), cognitive impairment (7), anxiety and depression (5,6,13). FoF has also been associated with potentially serious outcomes including reductions in physical activity, reduced ability to perform activities of daily living, abandon of social activities, worse quality of life, and increase in future falls (14,15).
Frailty is associated with falls (16) through a multicomponent causality including sarcopenia-related weakness, weight loss-related sarcopenia and low physical activity-related sarcopenia (17). Moreover, slowness and exhaustion can produce exercise and rehabilitation avoidance, increasing sarcopenia, balance impairment and falls. Although slowed gait speed, shorter stride length, and an increased double support phase, all related to the frailty phenotype, are associated with falls and FoF, there is no clear evidence that the frailty syndrome is associated with FoF. Indeed, few studies have analyzed the association between these two syndromes (18).
Methods
Objective
The main objective was to analyze the association between frailty and FoF in older adults in Spain.
Design
Cross-sectional analysis of the FISTAC Study (Identification of the Physical Attributes of the Fear of Falling Syndrome).
Population
Inclusion criteria were patients ≥70 years old, with at least a previous fall in the last year, who were visited at the Falls Unit of the Geriatrics Department, Complejo Hospitalario Universitario of Albacete (Spain). The complete protocol can be accessed in a previous publication (19). Written informed consent was necessary prior to enrollment in the study.
Fear of Falling assessment
FoF was assessed at baseline using two validated methods. The first one was the assessment of three questions that have been previously published (12) and used in other studies (7). Participants had to respond yes or no to the following questions: 1. Are you afraid of falling? 2. Do you limit any household activities because you are frightened you may fall? and 3. Do you limit any outside activities because you are frightened you may fall? If the response to any of the three questions was positive, the patient was classified as having FoF. These questions have been independently associated with activity restriction (12), balance and mobility impairment (7), and responses to similar questions correlate with the Falls Efficacy Scale.
The second method was the Falls Efficacy Scale International (FES-I) (20). This instrument evaluates the level of concern about FoF in several activities of daily living. It includes 16 items scoring between 1 and 4, being 1 the absence of concern and 4 the greatest concern. The scoring range of the complete scale is between 16 (no concern at all) and 64 (greatest concern). Scores between 16 and 19 reflect a low concern of FoF, between 20 and 27 moderate concern, and greater than 27 high concern (21).
Frailty assessment
We used the frailty phenotype criteria (22). 1. Unintentional weight loss ≥ 4.600 Kg or ≥ 5% of body weight in the last year. 2. Weakness as measured by grip strength, using a JAMAR® hand dynamometer, in the lowest 20%, adjusted for gender and body mass index, according to the Fried’s original cut-offs. 3. Poor energy and endurance, as indicated by self-reported exhaustion determined by two questions from the Center of Epidemiologic Studies Depression Scale. 4. Slowness, measured as the time taken to walk 4.0 meters, within the lowest 20th percentile and adjusted for gender and height, according to Fried’s original cut-offs. 5. Low physical activity level, calculating the number of kilocalories expended weekly from information given by the patient using the Calcumed® instrument, within the lowest quintile for each gender, with Fried’s original cut-off points. To construct the frailty phenotype variable, participants had to have valid values in at least 3 of the 5 criteria. Subjects were considered frail if three or more criteria were present and pre-frail if 1 or 2 were present. Frailty was analyzed as a dichotomic variable (yes/no), and every five criteria were also analyzed independently.
Study covariables
Age and gender were recorded, and chronic diseases were identified from the medical records of participants. Diseases were codified following the CIE-10 classification, and comorbidity was analyzed with the Charlson index. High comorbidity was considered when Charlson index score was equal or greater than 3 points. Cognitive status was determined with the Folstein´s Mini Mental State Examination (MMSE), and risk of depression with the Geriatric Depression Scale from Yesavage (GDS). Cognitive impairment was considered when MMSE was lower than 24 points and depression risk when GDS was higher than 4 points. Nutritional status was measured with the Mini Nutritional Assessment Short-Form (MNA®-SF), a 6-item scale that assesses nutritional risk, ranging from 0 (poorer nutritional state) to 14 (better nutritional state). Risk of malnutrition was considered with scores below 12 points. For study purposes, weakness and slowness were also categorized according to validated European Working Group on Sarcopenia in Older Adults (EWGSOP) criteria as follows: Weakness < 20 kg in women or < 30 kg in men, and slowness as usual gait speed < 0.8 m/s in both men and women.
Information sources
After the informed consent sign, information was collected through a single, one-to-one interview with the participant at the Falls Unit. The information was provided by the participant him/herself. The performance tests were conducted on the same day as the interview. The information on the participants’ chronic diseases was collected from the hospital medical records. Data were anonymized, codified and included in a data base for further analysis.
Ethics
This study complies with the Declaration of Helsinki and with the Organic Personal Data Protection Spanish Law 15/1999. The study was approved by the Institutional Review Board of the Albacete Health Area and the Clinical Research Committee of the Complejo Hospitalario Universitario de Albacete. All participants gave their written signed consent before being included in the study.
Statistics
A descriptive analysis of the subjects’ characteristics was performed using proportions and measures of central tendency and dispersion according to the nature of the variables. Subsequently, a bivariate analysis was performed using the Chi-squared tests and t-Student tests to determine differences in study variables according to the FoF status. Last, we conducted a multivariate analysis with logistic regression models to describe the variables independently associated with FoF. In the models we included progressively frailty, age, sex, Charlson index, MMSE, GDS and MNA®-SF. In these models, frailty was only analyzed as a dichotomic variable (yes/no) because the proportion of non-frail participants was very small our patients with recurrent falls. All data were stored and analyzed using the SPSS 20.0 software programme.
Results
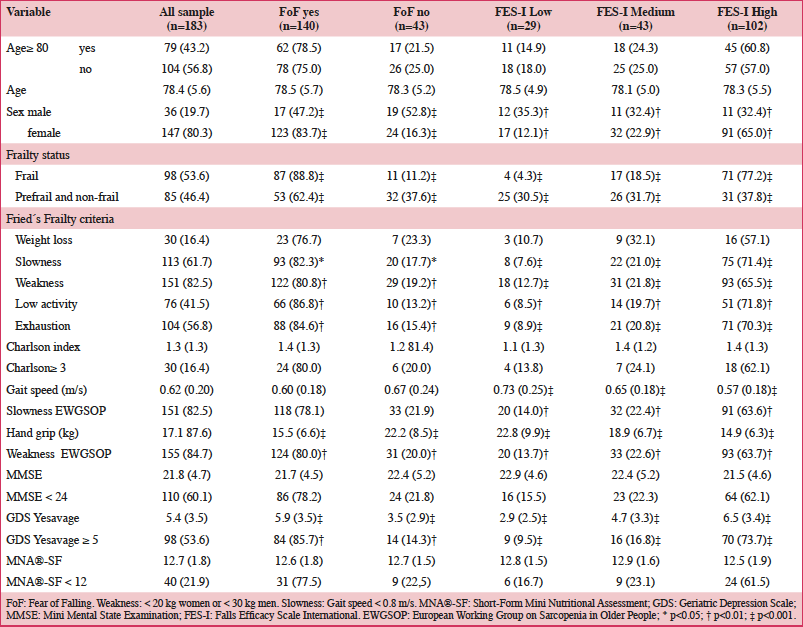
Table 1 presents the baseline characteristics of the global sample, and also categorized by FoF status. FoF was more prevalent in women than in men, but was not related neither to age nor comorbidity. Also, we couldn’t find association between neither nutritional status, nor cognitive decline with FoF.
FoF: Fear of Falling. Weakness: < 20 kg women or < 30 kg men. Slowness: Gait speed < 0.8 m/s. MNA®-SF: Short-Form Mini Nutritional Assessment; GDS: Geriatric Depression Scale; MMSE: Mini Mental State Examination; FES-I: Falls Efficacy Scale International. EWGSOP: European Working Group on Sarcopenia in Older People; * p<0.05; † p<0.01; ‡ p<0.001
However, there was a clear relationship between FoF and frailty. Based on the three questions, 88.8% of frail older adults presented FoF compared to 62.4% of those who were not frail. Furthermore, only 37.8% of non frail participants had a high concern of falling, compared to 77.2% of those who were frail measured with the FES-I. Analyzing every five components of the frailty phenotype, only weight loss was not associated with FoF. Slowness, weakness, and exhaustion were the three criteria with the highest association. In order to deepen in the association between frailty criteria and FoF, we also analyzed the association between EWGSOP criteria of weakness and slowness. More than 60% of participants with high concern of falling using the FES-I presented slowness and weakness when using EWGSOP cut-points, compared to 30% of those who did not meet EWGSOP criteria.
Regarding depression, 85.7% of participants with a GDS score greater than 4 presented FoF compared to 65.9% of those with lower GDS scores. Only 40.5% of those without depression risk had a high concern of falling, compared to 73.7% of those with depression risk.
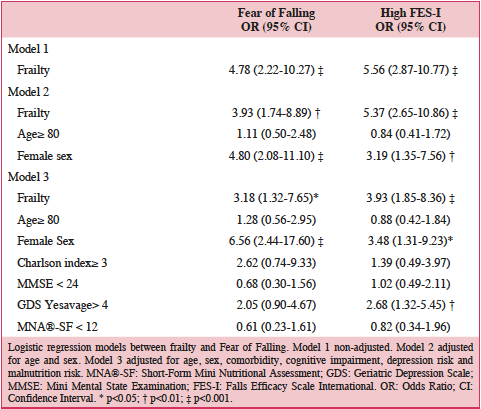
Finally, we designed three progressive logistic regression models in order to determine the adjusted relationship between frailty and FoF. Frail participants had an adjusted risk of FoF that was 3.18 (95% CI 1.32 to 7.65) higher compared to those who were not frail, assessed with the three questions. Similarly, frail participants had a 3.93 (95%CI 1.85 to 8.36) increased adjusted risk of presenting high concern of falling when using the FES-I scale, when compared to those non frail. Only female sex and depression risk were also associated to FoF in the final adjusted models.
Logistic regression models between frailty and Fear of Falling. Model 1 non-adjusted. Model 2 adjusted for age and sex. Model 3 adjusted for age, sex, comorbidity, cognitive impairment, depression risk and malnutrition risk. MNA®-SF: Short-Form Mini Nutritional Assessment; GDS: Geriatric Depression Scale; MMSE: Mini Mental State Examination; FES-I: Falls Efficacy Scale International. OR: Odds Ratio; CI: Confidence Interval. * p<0.05; † p<0.01; ‡ p<0.001.
Discussion
The main conclusion of our study is that frailty is independently associated to FoF in older adults with a previous fall. Frail older adults have a three to four-fold an adjusted increased risk of presenting FoF, depending on the instrument used to asses this syndrome. Furthermore, neither comorbidity, nor cognitive status, nor nutritional status are associated with this entity, and only female sex and depression risk are.
Frailty, one of the cornerstones of Geriatric Medicine (1), is very common among older adults who fall (16). In the Beijing Longitudinal Study of Aging, the Frailty Index was associated with an increased risk of recurrent falls (OR 1.54) (23), and in the Study of Osteoporotic Fractures (16), frail women had a higher age-adjusted risk of recurrent falls (OR 2.4). In our sample 53.6% of the participants were frail, confirming this association.
Falls and FoF share a common characteristic, namely mobility impairment (6), and both are associated with physical performance elements such as balance and strength (14). In a recent review, FoF was robustly associated with impaired physical function in community-dwelling older adults (24). In our study, four components of the Fried´s frailty criteria were associated with FoF, namely slowness, weakness, low physical activity and exhaustion, and only one, weight loss was not. Austin et al. found that older adults with FoF presented longer time to complete the Timed up and go test compared to those without fear (10.1 vs 9.0 seconds), and more commonly had a lack of physical activity (32.1% vs 19.5%), both components of the frailty syndrome, in agreement with our data (7).
It has been described that gait abnormalities can predispose an individual to reduced mobility and an increased likelihood of falling, and that FoF influences spatial and temporal gait parameter changes in older adults. Slower gait speed, shorter stride length, increased stride width, and prolonged double limb support time, have all been associated with a preexisting FoF (25). However, other authors have proposed that low gait speed associated with FoF, may be a useful adaptation to optimize balance, rather than a sign of decreased balance control. The reason is because the ability to attend to a secondary task during walking is not influenced by FoF (10). FoF is associated with an increase in gait variability and a recent meta-analysis concluded that the augmentation in stride time variability related to FoF should be considered as a biomarker of impairments of higher-level gait control (26). However, the authors discussed that the mixed results obtained in other studies suggest that cortical gait control impairment related to FoF is not as simple as believed and may require additional disorders, such as falling, to induce significant changes in gait control.
Knee muscle strength is an important and independent determinant of falls and the level of FoF in individuals with Parkinson´s Disease (27). In this population, reduced lower extremity muscle strength was associated with recurrent falls, and also with a lack of confidence in performing standing or walking activities. In our study there was a strong association between grip strength and FoF, probably suggesting that a global state of sarcopenia is associated with FoF, and not only lower limb muscle mass decline. Improving balance, gait stability and knee muscle strength could be crucial in promoting balance confidence (27).
The main limitation of our study is the cross-sectional design. We can´t demonstrate causality in the relationship between frailty and FoF, and longitudinal studies should be necessary to confirm this association. However, the rationale of the demonstrated association between frailty and falls makes also very plausible the association between frailty and FoF. In our study there were a high percentage of women (80%) and most of the participants presented FoF (76%). The relationship between female sex, frailty and falls is well described in the literature and for this reason in order to avoid bias, we included in our models sex as an important variable.
Conflict of Interest
There is no conflict of interest for any author.
Declaration of sources of funding
This work was supported by Instituto de Salud Carlos III, Ministerio de Economía y Competitividad, and Fondo Europeo de Desarrollo Regional (European Union) [grant numbert PI12/02180], and CIBERFES, Instituto de Salud Carlos III, Spain.
References
1. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013;14:392-7.
2. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am GeriatrSoc 2012;60:1487-92.
3. Romero Rizos L, Abizanda Soler P. Frailty as a predictor of adverse events in epidemiological studies: literature review. Rev Esp Geriatr Gerontol 2013;48:285-9.
4. Tinetti ME, Richman D, Powell L. Falls efficacy as a measure of fear of falling. J Gerontol 1990;45:239–43.
5. Arfken CL, Lach HW, Birge SJ, Miller JP. The prevalence of fear of falling in elderly persons living in the community. Am J Public Health 1994;84:566-70.
6. Friedman SM, Munoz B, West SK et al. Falls and fear of falling: Which comes first? A longitudinal prediction model suggests strategies for primary and secondary prevention. J Am Geriatr Soc 2002;50:1329-35.
7. Austin N, Devine A, Dick I, Prince R, Bruce D. Fear of falling in older women: a longitudinal study of incidence, persistence, and predictors. J Am Geriatr Soc 2007;55:1598-603.
8. Scheffer AC, Schuurmans MJ, Van Dijk N, Van der Hooft T, de Rooij SE. Fear of falling: measurement strategy, prevalence, risk factors and consequences among older persons. Age Ageing 2008;37:19-24.
9. Murphy SL, Dubin JA, Gill TM. The development of fear of falling among community-living older women: Predisposing factors and subsequent fall events. J Gerontol A Biol Sci Med Sci 2003;58A:M943-M947.
10. Reelick M.F, Van Iersel MB, Kessels RP, Rikkert MG. The influence of fear of falling on gait and balance in older people. Age Aging 2009;38:435-440.
11. Lach HW. Incidence and risk factors for developing fear of falling in older adults. Public Health Nurs 2005;22:45–52.
12. Bruce DG, Devine A, Prince RL. Recreational physical activity levels in healthy older women: the importance of fear of falling. J Am Geriatr Soc 2002;50:84-9.
13. Van Haastregt JC, Zijlstra GA, van Roosum E, van Eijk JT, Kempen GI. Feelings of anxiety and symptoms of depression in community-living older persons who avoid activity for fear of falling. Am J Geriatr Psychiatry 2008;16:186-93.
14. Deshpande N, Metter EJ, Lauretani F, Bandinelli S, Guralnik J, Ferrucci L. Activity restriction induced by fear of falling and objective and subjective measures of physical function: a prospective cohort study. J Am Geriatr Soc 2008;56:615.
15. Trombetti A, Reid KF, Hars M, et al. Age-associated declines in muscle mass, strength, power, and physical performance: impact on fear of falling and quality of life. Osteoporos Int 2016;27:463-71.
16. Ensrud KE, Ewing SK, Taylor BC, et al. Frailty and risk offalls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci 2007;62:744-51.
17. Morley JE. Frailty, falls, and fractures. J Am Med Direct Assoc 2013;14:149-51.
18. Delbaere K, Crombez G, Vanderstraeten G, Willems T, Cambier D. Fear-related avoidance of activities, falls and physical frailty. A prospective community-based cohort study. Age Ageing 2004;33:368-73.
19. Esbrí Víctor M, Huedo Rodenas I, López Utiel M, et al. Rationale, design and methodology of physical attributes identification of the fear of falling syndrome (FISTAC study). Rev Esp Geriatr Gerontol 2016;52:80-6.
20. Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005;34:614-9.
21. Delbaere K, Close JCT, Mikolaizak AS, Sachdev PS, Brodaty H, Lord SR. The Falls Efficacy Scale International (FES-I). A comprehensive longitudinal validation study. Age Ageing 2010;39:210-6.
22. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in Older Adults: Evidence for a Phenotype. J Gerontol A Biol Sci Med Sci 2001;56A:M146-M156.
23. Fang X, Shi J, Song X, et al. Frailty in relation to the risk of falls, fractures, andmortality in older Chinese adults: Results from the Beijing longitudinal study of aging. J Nutr Health Aging 2012;16:903-7.
24. Denkinger MD, Lukas A, Nikolaus T, Hauer K. Factors associated with fear of falling and associated activity restriction in community-dwelling older adults: a systematic review. Am J Geriatr Psychiatry 2015;23:72-86.
25. Chamberlin ME, Fulwider BD, Sanders SL, Medeiros JM. Does fear of falling influence spatial and temporal gait parameters in elderly persons beyond changes associated with normal aging? J Gerontol A Biol Sci Med Sci 2005;60A:1163-7.
26. Ayoubi F, Launay CP, Annweiler C, Beauchet O. Fear of falling and gait variability in older adults: a systematic review and meta-analysis. J Am Med Dir Assoc 2015;16:14-9.
27. Mak MKY, Pang MYC, MOK V. Gait difficulty, postural instability, and muscle weakness are associated with fear of falling in people with parkinson’s disease. Parkinsons Dis 2012;2012:901721.