Rosario Sakamoto1, Barbara J Cherry2, Stephanie Vaughn1
1. School of Nursing, California State University, Fullerton, Fullerton, CA, USA , 2. Psychology Department, California State University, Fullerton, CA, USA
Corresponding to: Rosario Sakamoto, DrPH, MSN, CCRN, NP-BC, Assistant Professor, Coordinator, Robust Aging Program, School of Nursing, California State University, Fullerton, 800 North State College Blvd, Fullerton, CA. 92831, USA
Tel. 657-278-7649,| Tel. 657- 278- 3392, Fax 657-278-3338, E-mail: rsakamoto@fullerton.edu
Care Weekly 2019;
Published online Februay 20, 2019, http://dx.doi.org/10.14283/cw.2019.1
Abstract
Objectives: Explore the effects of vitamin D supplementation on global cognition, executive function and episodic memory among older community dwellers.
Design: Parallel group, double-blind pretest-posttest placebo-controlled randomized pilot study.
Setting/Participants: Robustly aging older community dwellers: Osher Lifelong Institute members of the California State University, Fullerton. Sample size: 61, 12 with intervention.
Intervention: Vitamin D3 5000 IU administered orally daily for six months. Baseline serum 25OHD and post six-month supplementation measured likewise, cognitive testing done.
Measurements: Chemiluminescence LIASON® assay was used for determination of serum 25OHD levels. Mini-Mental State Exam (MMSE) assessed global cognition, executive function with Letter-Number Sequencing and Stroop Color-Word tests, episodic memory with immediate and delayed Logical Memory tests.
Randomization/Blinding: The twelve participants were randomly assigned to treatment or placebo groups (7 with active pills, 5 with placebo). Both participants and clinic nurses were blinded to results of randomization.
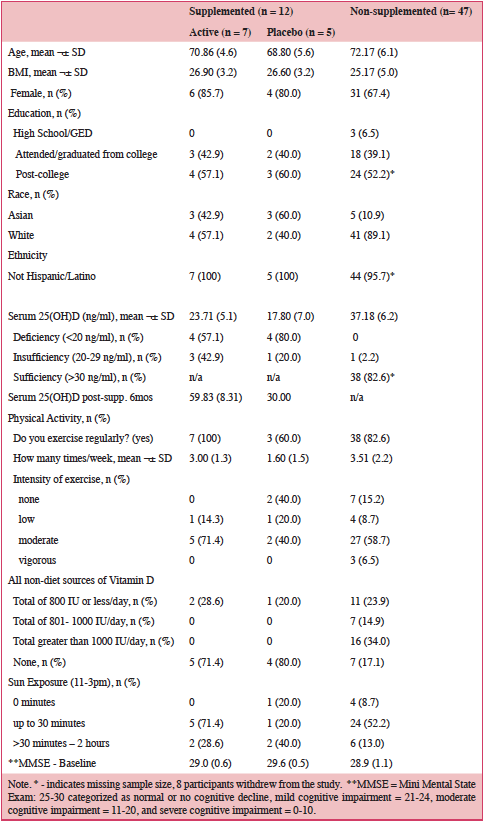
Results: The demographics revealed the following: Age 60 – 88 years, mean =70 years, BMI mean = 26, with more females (78%) than males (22%). Individuals were predominantly White (62%), highly educated with post-college education (56%), and physically, moderately active. Serum vitamin D levels increased significantly from baseline mean 24ng/ml (60nmol/L) to 60ng/ml (150nmol/L). Six months’ vitamin D supplementation showed significant improvement in global cognition for the treatment versus placebo groups, p = 0.04, with a trend for improvement in Stroop measures, p’s = .097; .093. No adverse events or side effects, high compliance with taking pills and well tolerated.
Conclusion: Healthy older individuals who had intact cognition, supplemented with a high dose of vitamin D3 (cholecalciferol) and followed for six months showed improvement on the global mental status and trended towards improvement in executive function. Vitamin D3 (cholecalciferol) 5000 IU daily increases serum vitamin D levels that reduced vitamin D deficiency, and may improve global cognition but not executive function or memory.
Key words: Vitamin D supplementation, hypovitaminosis D, vitamin D deficiency, global cognition, serum 25(OH)D.
Introduction
Approximately 70-90% of older adults, 65 years and above, have cognitive difficulties and also suffer from vitamin D deficiency (1). Vitamin D (cholecalciferol) is one of the dietary factors that has been suggested (2) to improve many vascular risks such as the slowing of brain tissue plaque and tangle formation, which may delay the onset of cognitive decline or prevent the progression of cognitive dysfunction. Observational studies showed associations between vitamin D deficiency with both Alzheimer’s disease (AD) and cognitive impairment (3). Not a simple vitamin but a hormone, vitamin D has multiple biological functions which influence hundreds of genes; that is, most cells contain a nuclear vitamin D receptor (VDR) that interacts with cellular processes (4). Vitamin D deficiency studies showed linkage with an increased risk of age-related chronic diseases including AD through neuronal loss (2, 4).
Although some studies have shown valuable relationships between vitamin D status and cognitive decline, (3, 5), others have demonstrated conflicting results (6, 7). Effective pharmacological treatment and preventative interventions remain lacking in preventing cognitive decline or delaying the progression of AD or any type of dementia (8). This lack of clear interventions leads to a growing interest in studying preventive strategies such as an effective nutritional lifestyle through adequate dietary practices. The current interpretation of vitamin D study results is unclear due to several methodological flaws such as inadequate dosages, small sample sizes, and short duration of studies (9). Despite conflicting results, studies have consistently shown vitamin D’s positive influence on cognition such that researchers suggest the need for randomized trials at this time (10). Hence, this study was implemented to meet the need to engage in a placebo-controlled randomized interventional study. The purpose was to explore the feasibility of whether vitamin D3-5000 International Units (IU) oral supplementation daily for six months would influence cognitive function among healthy older community-dwellers. Our specific objective was to determine if six months’ vitamin D supplementation among those with existing hypovitaminosis D influence cognitive function particularly memory and executive function domains.
Methods
Study Design
This pilot study was a six-month randomized trial– a pre-post-supplementation, parallel- design, non-inferiority concept to assess the effects of high dose 5000 IU vitamin D3 (cholecalciferol, manufacturer: Bio-Tech Pharmacal, Inc.) on cognition. The study was a double-blinded trial– both the nurses who administered the vitamins, cognitive testers and the participants were not aware of group assignment.
Eligible participants for supplementation after the randomization process were given the corresponding supply of labeled cholecalciferol vitamin D3 bottle of pills. Those who randomly chose #1 had the 100 active pills and those who randomly chose #2 had 100 placebo capsules for their three months’ supply. Monitoring consisted of either a phone call or email or a brief clinic visit with a self-report on the compliance of taking vitamin D supplements, and pill count at three months’ and again at six months’ follow up. The site of refills and study was at the university’s Robust Aging Program clinic.
Participants
Participants were members of the California State University’s Osher Lifelong Learning Institute (OLLI-CSUF) who were attendees of the Ruby Gerontology Center’s activities. Recruitment included those 60 to 90 years old, who had vitamin D insufficiency or deficiency or were not aware of their vitamin D status. Participants were not taking any vitamin D supplementation >1000 IU (International Units) for at least three months prior to the study. Exclusion assessment was self-reported included a diagnosis of intellectual disability or dementia (moderate to severe stages), a history of neurological damage, including but not limited to cerebral vascular disease; previous head injury, stroke, or coronary artery bypass or neurosurgical procedure, and who are non-English speakers, or unable to follow basic cognitive testing instructions. Participants with known bone disorders and hypercalcemia, cancers (except skin cancers) within past 10 years, kidney stone disease or history of kidney stones, renal failure, chronic liver disease, alcoholism, and uncontrolled diabetes were also excluded. Those taking anti-dementia drugs (anti-cholinergic, i.e. Memantine) and other medications: phenytoin or phenobarbital or other drugs interfering with vitamin D metabolism were excluded as well. Inclusion and exclusion criteria were followed to ensure safety in taking six months’ supplementation above the current Institute of Medicine recommendations (11). Those taking anti-dementia drugs were excluded as well. Out of 61 participants recruited, 12 individuals with 25OHD < 30ng/ml (< 75nmol/L) levels qualified for supplementation. We originally plan to include all 59 participants, 47 with sufficient levels as control. However, for more efficient use of limited resources and in accordance to current recommendations to do studies that include those with vitamin D insufficiency/deficiency, we decided to study the 12 participants that met the criteria.
Interventions
This study entailed three face-to-face visits: baseline, after three and six months’ supplementation. Potential participants were screened for eligibility and after consents were signed, they became active participants. A health history and demographic forms were completed to assess health issues, medical history, medication use, and lifestyle. Vital signs including anthropometric measurements: height/weight, waist circumference, and BMI were measured. The university’s Internal Review Board approved the study protocol. Blood serum vitamin D 25OHD levels were drawn at the university’s Student Health Center laboratory pre and post-supplementation. The total vitamin D, LIASON® assay was done by a direct competitive chemiluminescence immunoassay (CLIA). The derived functional sensitivity from the regression equation from samples was <4.0 ng/mL with intra-assay coefficient variation (CV) of 3.8% at 8ng/ml (20nmol/L), and inter-assay of 12.2% CV (12).
The Mini-Mental State Exam (MMSE) was used to measure global cognition. This test has been widely used for global cognition testing for a rapid detection of changes in cognitive function and its severity: maximum score = 30, with 25-30 considered normal (13). The tests for memory consisted of the Wechsler Memory Scale-Third Edition (WMS III) with subtest scores of the primary indexes across age groups: .74 to .93, with a .81 median reliability, reported to be acceptable to excellent reliabilities (14). The subsets used were: Logical Memory I Immediate Recall with Logical Memory II Delayed Recall, Letter-Number Sequencing. A Stroop Color-Word Test was used to assess executive functioning (15). Both randomly assigned groups received the same interventions done at baseline and after six months.
Sample Size and Data Analysis
We determined through G-power analysis (16) that we needed 64 participants for medium effect with a two-tailed a of 0.05, with a power of 80% significance level for a comparison of two independent groups. Adjusting for 20% attrition rate we targeted to recruit 80 participants with 40 active and 40 placebo participants. After several attempts to recruit more participants, the actual sample size differed from the original intended sample calculation. To allocate the participants, an individual blindly chose a number inside a bag with equal numbers of active and placebo small slips of paper, i.e., two slips of paper with #1 (active) and two slips of paper with #2 (placebo) until all 12 qualified participants picked a number. Allocation was double blind; both the nurse administering the procedure did not know what numbers had been picked, and participants did not know what numbers were in the bag.
Analysis entailed chi-square tests for the categorical variables when comparing baseline characteristics between groups, utilizing SPSS version 24 (SPSS Inc. Chicago, IL, USA). Based on participants’ serum 25OHD levels, we categorized participants into three categories: deficient <20ng/ml (8nmol/L), insufficient 21-29ng/ml (8.4 -11.6nmol/L) and sufficient >30ng/ml (75nmol/L). We used t-tests to compare the differences between the supplemented (active) and non-supplemented (placebo) groups. To analyze the effects of the supplementation for active versus placebo groups, we used repeated measures analysis of variance (ANOVA). Our primary analysis involved all patients who were randomly assigned to either the active or placebo group.
To check if we met our specific objective of determining effects of supplementation between the two groups, we also calculated effect sizes and estimated sample sizes needed to test for significant differences between the allocated groups using 95% confidence intervals for these estimates pre and post-tests.
Results
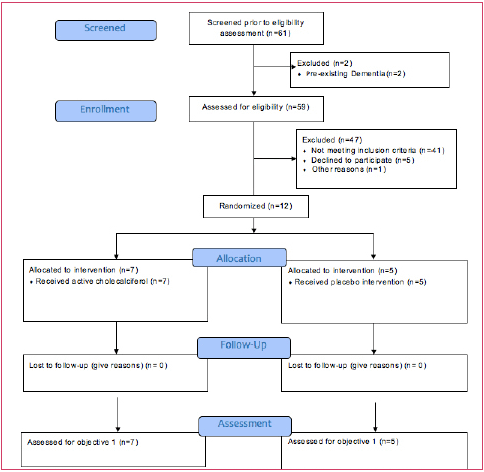
A total of 61 were screened for eligibility assessment and 59 were eligible and enrolled. However, 47 participants were excluded with 41 not meeting criteria for supplementation and six lost to attrition. The remaining twelve participants were allocated randomly assigned to the active group (7) and placebo group (5). No one was lost to follow-up at three or six months as shown in CONSORT guidelines flowchart (17), see Figure 1. Recruitment started the middle of March, 2017 to June 2017, follow-up started June, 2017 to May, 2018. The pilot study ended with small sample size due to less volunteers and/or ineligible for supplementation volunteers despite recruitment from outside nearby medical clinics within limited timeline/resources.
Adapted from: Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355.
Table 1.0 shows the average serum 25OHD for all participants was 27ng/ml(68nmol/L). Supplementation of 5000 IU/day significantly increased serum vitamin D levels: 24ng/ml (60nmol/L) to 60ng/ml (150nmol/L) – active pills; 18ng/ml (45nmol/L) to 30ng/ml (75nmol/L) – placebo pills after six months. The supplemented and non-supplemented were similar in educational levels, exercise and sun-seeking behaviors and baseline MMSE scores (≥29).
Mixed repeated measures ANOVAs were performed using two (active, placebo) by two (pretest, posttest) independent variables with each cognitive measure as the dependent variable. Logical Memory I, Logical Memory II and Letter-Number-Sequencing showed no significant main effects or interactions. Results for MMSE showed no main effect for group, F (1,10)=1.10, MSe=.576, p=.32, but a significant effect for pretest-posttest scores, F (1,10)=5.56, MSe=.476, p=.04 and a group by pretest-posttest interaction, F (1,10)=5.56, MSe=.476, p=.04, (Table 2.). No main effects were found for Stroop Color-Word (group F<1, or pre-post test scores F (1,10)=2.87, MSe=.142.519, p=.121) but a slight trend for the interaction, F (1,10)=3.36, MSe=166.519, p=.097. Stroop C also showed no main effects for group or pretest-test scores, F’s < 1, but again a trend for the interaction, F (1,10)=3.44, MSe=64.630, p=.093.
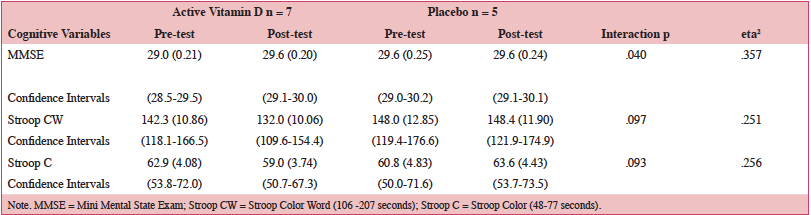
Table 2. Pre- and Post-test Means (SE) and 95% Confidence Intervals for MMSE and Stroop for Active versus Placebo Groups
Discussion
Results of this pilot study determined that vitamin D supplementation of 5000 IU/day increased serum 25OHD levels significantly in all of the participants with the active but not placebo pills. Despite higher supplementation dosage than the current Institute of Medicine’s (IOM) recommendations (11), such dosage was well tolerated. There were no adverse events or reactions observed in either group. Study results showed that among healthy older adults taking vitamin D (5000 IU) supplementation for six months, global cognition improved with a trend towards improvement in executive function, but not episodic memory.
Limitations of our study included small sample size, particularly for the supplemented groups. Most participants were very high-functioning older community-dwellers with access to healthcare and other resources which could have skewed results. OLLI member- participants were mainly Caucasian older adults with high global cognitive levels, highly educated and physically active individuals living in sunny Orange County, CA.
While current literature shows many studies demonstrating that hypovitaminosis D equates to poor global cognition (3); there is a paucity in interventional studies showing that vitamin D supplementation improves global cognitive performance in highly functioning older adults. Vitamin D’s purported neuroprotective effects on global cognition and executive function could be due to vascular effects through calcium homeostasis (4); where chronic hypovitaminosis D may promote AD (4, 5). By including participants with insufficient/deficient levels, and a higher dose of vitamin D3 for longer duration and maintaining their sun exposure activities among older adults, this pilot study of supplementation can be implemented in the prevention of cognitive decline of at-risk general elderly population. It is reasonable to assume that we followed current vitamin D supplementation RCT recommendations in predicting health outcomes (18).
The effect size (eta2) for each of our cognitive measures in Table 2 was large. A consideration of the sample size needed to potentially demonstrate significant differences for active versus placebo groups with 80% power would be a minimum of 14 per group for Stroop CW and 11 per group for Stroop C. Variability in our measures was controlled for by using well established cognitive assessments as well as trained testers. Given time constraints and other limited resources, it was not possible to augment or investigate this further. Strengths of our study included high dosage supplementation with high compliance over six months’ duration in participants with hypovitaminosis D, and use of a variety of well-established cognitive tests. In sum, vitamin D supplementation 5000 IU for six months improved global cognitive performance, with improved serum vitamin D status.
Future Recommendations
Future research should consider older adults with different socio-economic status and ethnic backgrounds as well as test in locations with less ample sunlight. Potential confounding variables that might be considered would be sun exposure and exercise. Study results inform the public that vitamin D supplementation is safe above current IOM recommendations. Randomized placebo-controlled studies with larger, more diverse samples are needed to better understand vitamin D’s effect on specific cognitive domains. Alternate versions of the cognitive tests may have counterbalanced any practice effects. Also, other biomarkers could have been tested: PTH and Ca levels. Future research should also determine repletion at appropriate timing in one’s lifespan with clear dosage for both prevention and maintenance of cognitive function.
Acknowledgements
We would like to thank Dr. Richard Boucher, Chief Staff Physician of the California State University, Fullerton Student Wellness Health Services for his support in making the University Laboratory the site for serum Vitamin-D testing. This made it convenient for the participants to have had their blood samples drawn within the campus which contributed to the success of this Vitamin D-Cognition study.
Participating Center
The Ruby Gerontology Center and Osher Lifelong Learning Institute, CSUF for their support and cooperation in making this study possible.
Potential conflicts of interest
None
Study Registration
IRB_HSR #17_0019 California State University, Fullerton
Funding
California State University, Fullerton: Intramural Junior Faculty Grant. CSUF had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript.
References
1. Annweiler C, Dursun E, Féron F, Gezen-Ak D, Kalueff AV, Littlejohns T, et al. ‘Vitamin D and cognition in older adults’: updated international recommendations. Journal of Internal Medicine. 2015;277(1):45-57.
2. Millet P, Landel V, Virard I, Morello M, Feron F. [Role of vitamin D in the physiopathology of neurodegenerative diseases]. Biologie aujourd’hui. 2014;208(1):77-88.
3. Feart C, Helmer C, Merle B, Herrmann FR, Annweiler C, Dartigues JF, et al. Associations of lower vitamin D concentrations with cognitive decline and long-term risk of dementia and Alzheimer’s disease in older adults. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2017;13(11):1207-16.
4. Annweiler C, Schott AM, Berrut G, Chauvire V, Le Gall D, Inzitari M, et al. Vitamin D and ageing: neurological issues. Neuropsychobiology. 2010;62(3):139-50.
5. Miller JW, Harvey DJ, Beckett LA, Green R, Farias ST, Reed BR, et al. Vitamin D Status and Rates of Cognitive Decline in a Multiethnic Cohort of Older Adults. JAMA neurology. 2015;72(11):1295-303.
6. Rossom RC, Espeland MA, Manson JE, Dysken MW, Johnson KC, Lane DS, et al. Calcium and vitamin D supplementation and cognitive impairment in the women’s health initiative. J Am Geriatr Soc. 2012;60(12):2197-205.
7. Stein MS, Scherer SC, Ladd KS, Harrison LC. A randomized controlled trial of high-dose vitamin D2 followed by intranasal insulin in Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2011;26(3):477-84.
8. Fink HA, Jutkowitz E, McCarten JR, Hemmy LS, Butler M, Davila H, et al. Pharmacologic Interventions to Prevent Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer-Type Dementia: A Systematic Review. Ann Intern Med. 2018;168(1):39-51.
9. Pludowski P, Holick MF, Grant WB, Konstantynowicz J, Mascarenhas MR, Haq A, et al. Vitamin D supplementation guidelines. J Steroid Biochem Mol Biol. 2018;175:125-35.
10. Annweiler C, Beauchet O. Vitamin D-mentia: randomized clinical trials should be the next step. Neuroepidemiology. 2011;37(3-4):249-58.
11. IOM. Dietary Reference Intakes for Vitamin D and CalciumDietary reference intakes for calcium and vitamin D. Ross A, Taylor C, Yaktine A, Del Valle H, editors. Washington, D.C.: The National Academies Press; 2011.
12. Le Goff C, Cavalier E, Souberbielle JC, González-Antuña A, Delvin E. Measurement of circulating 25-hydroxyvitamin D: A historical review. Practical Laboratory Medicine. 2015;2:1-14.
13. Folstein MF, Folstein SE, McHugh PR, Fanjiang G. Mini-Mental State Examination. In: Albanese MA, Ward SB, editors. MMSE1975.
14. Wechsler D. Wechsler Memory Scale III. In: D’Amato RC, Reynolds CR, editors. 1997.
15. Trenerry MR, Crosson B, DeBoe J, Leber WR. Stroop Neuropsychological Screening Test. In: Meier M, Reynolds CR, editors. SNST. Lutz, FL: Psychological Assessment Resources; 1989.
16. Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods. 2009;41(4):1149-60.
17. Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Bmj. 2016;355:i5239.
18. Grant WB, Boucher BJ, Bhattoa HP, Lahore H. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol. 2017.